It has been found that the pH of a 0.01 M solution of an organic acid is 4.15 . Calculate the concentration of the anion, the ionization constant of the acid and its pKa .

Recent trends on density functional theory–assisted calculations of structures and properties of metal–organic frameworks and metal–organic frameworks-derived nanocarbons | Journal of Materials Research | Cambridge Core
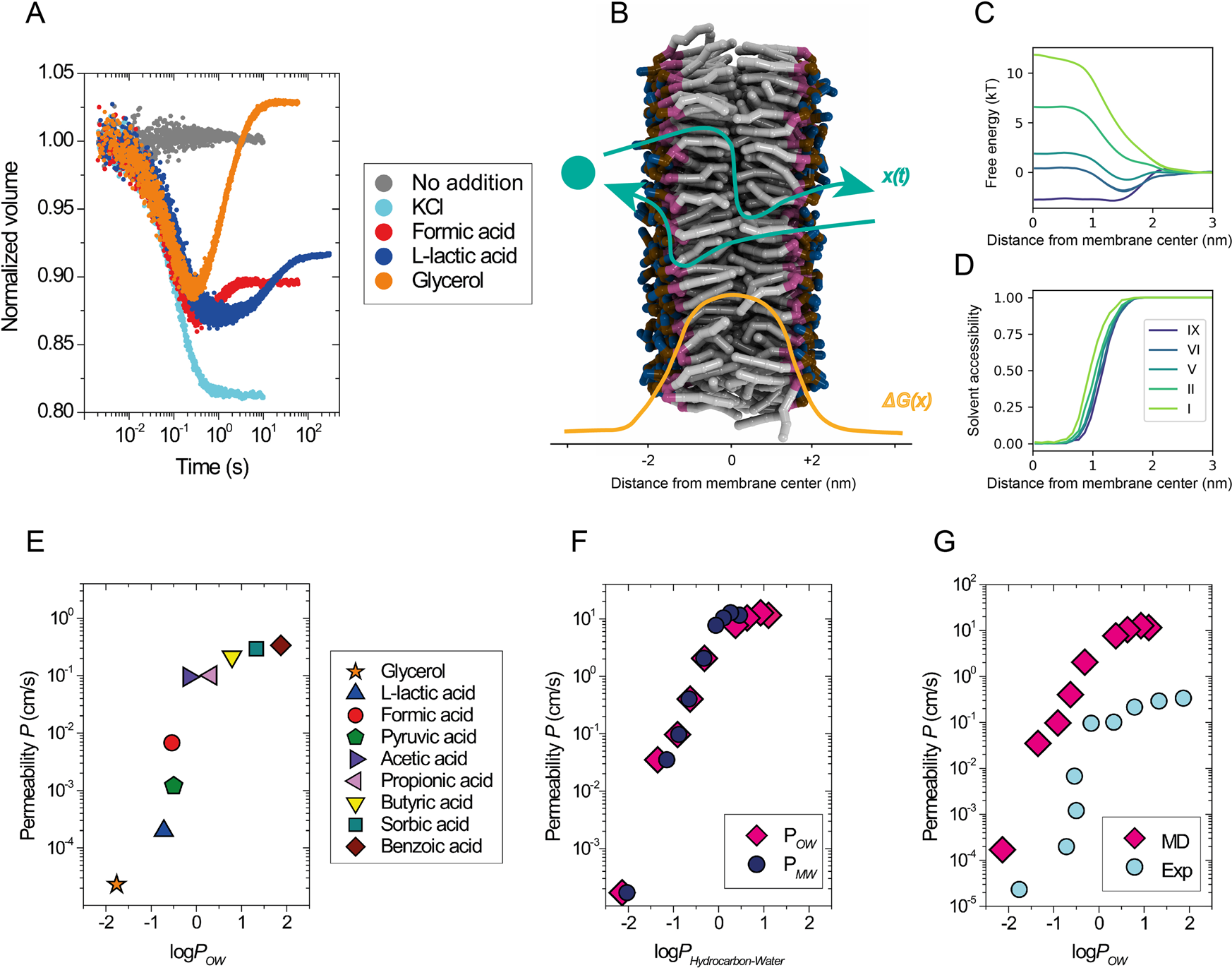
Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes | Nature Communications

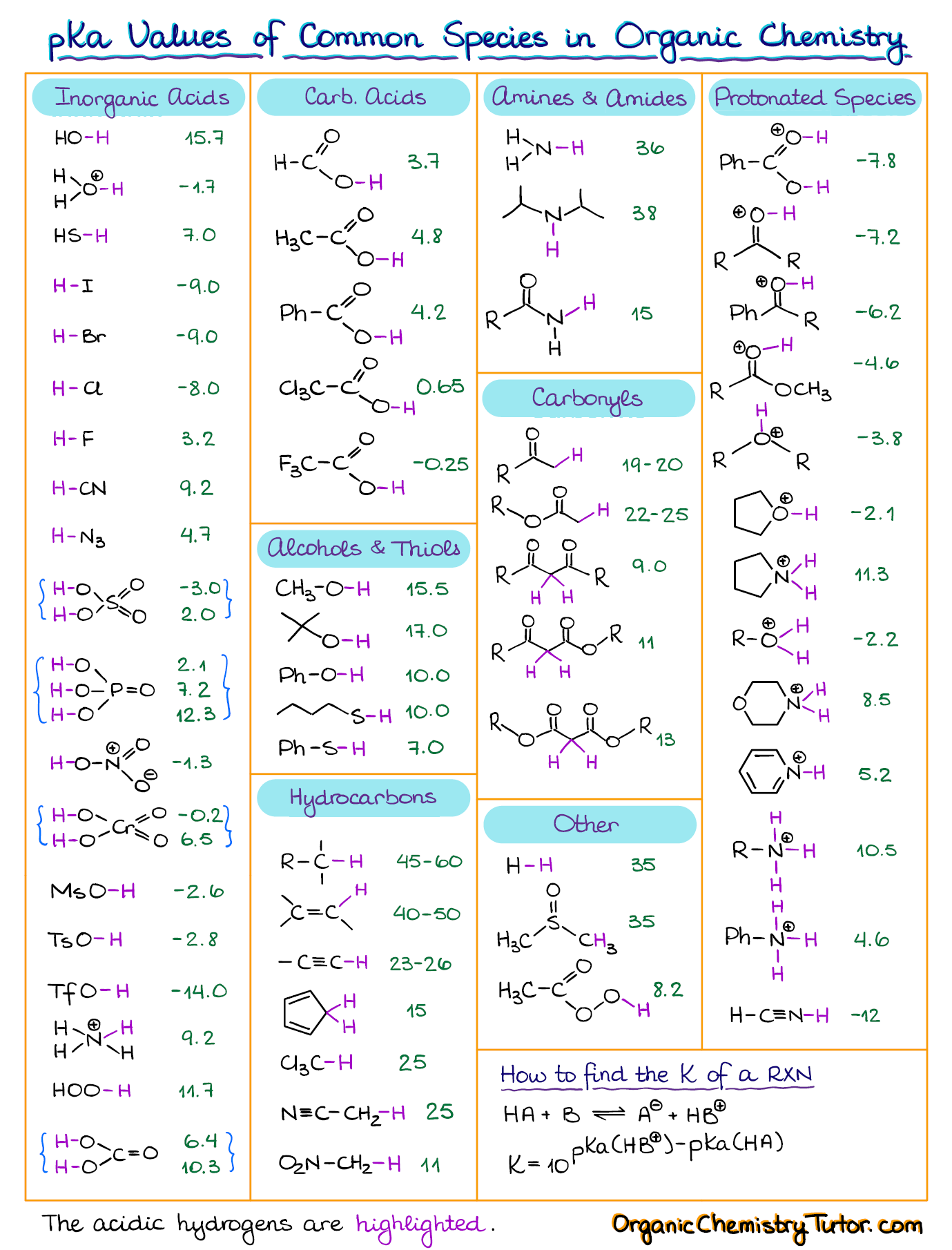
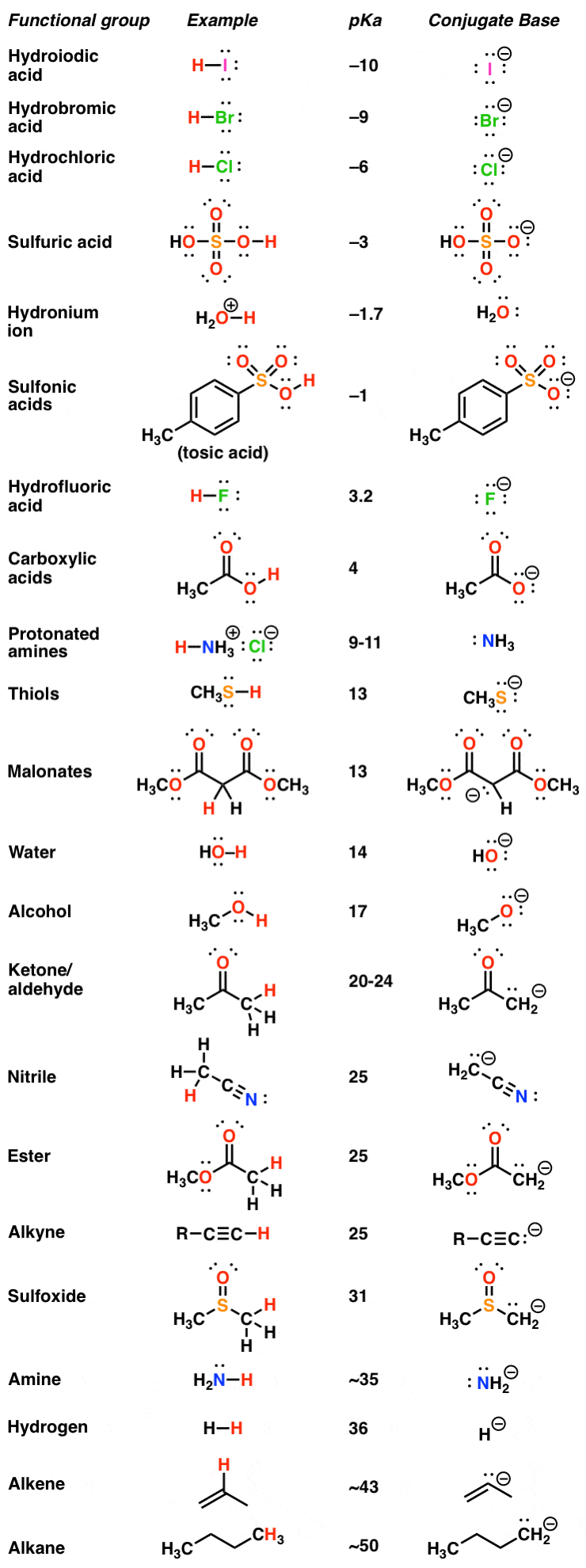
3.3: pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome - Chemistry LibreTexts

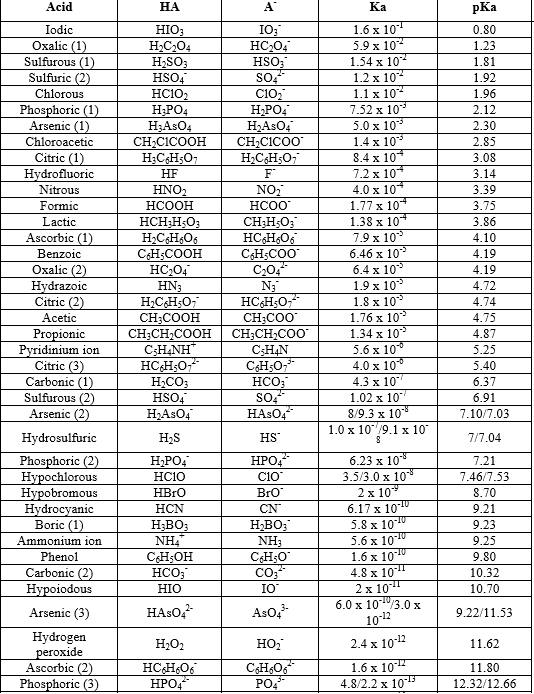
How to Identify the Major Species in a Mixture of Weak and Strong Acids or Bases | Chemistry | Study.com
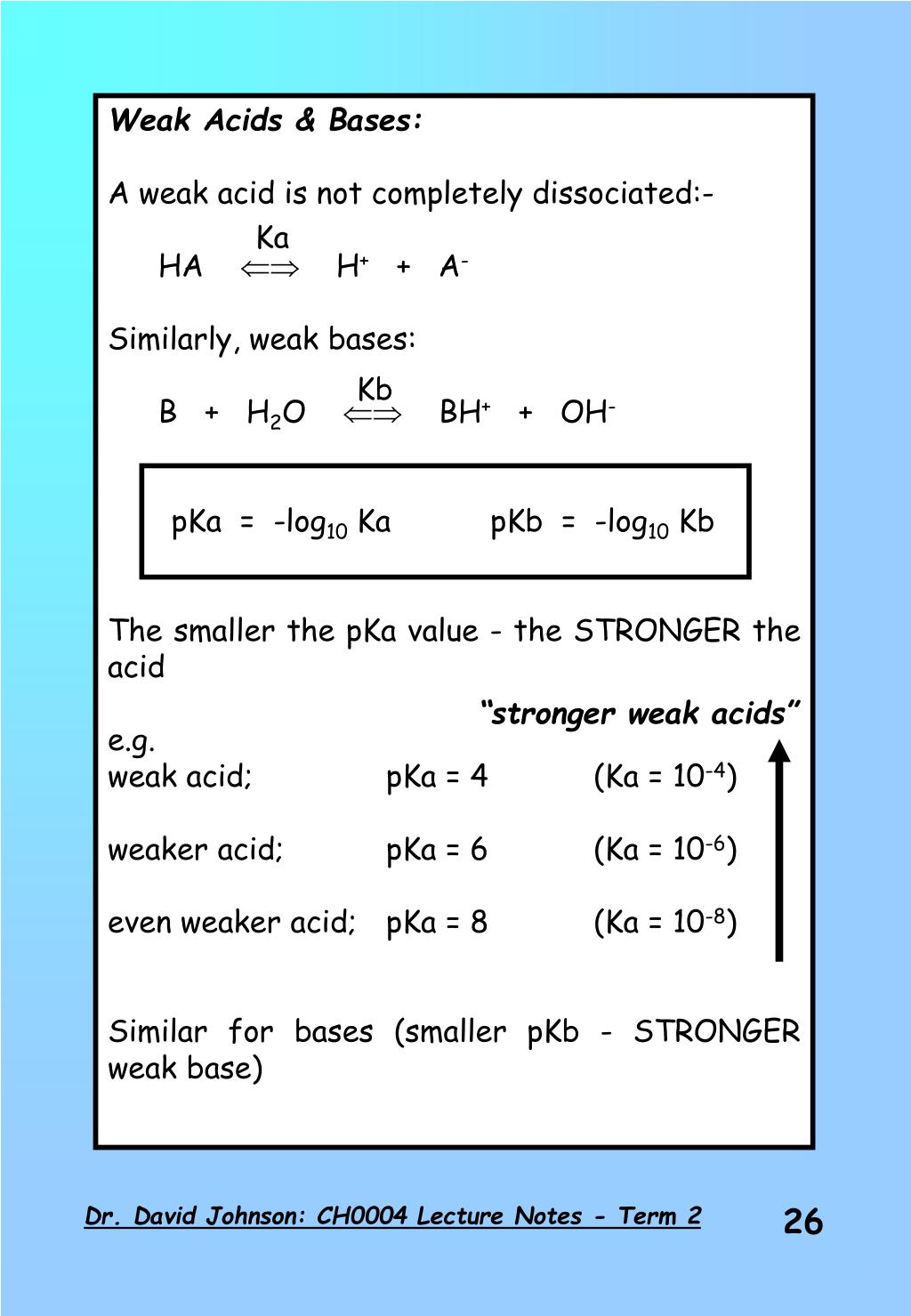
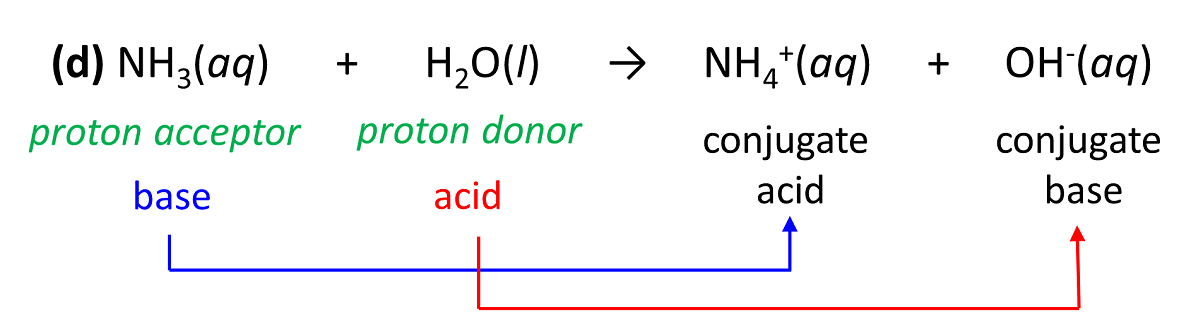
PPT - Weak Acids & Bases: A weak acid is not completely dissociated:- HA H + + A - PowerPoint Presentation - ID:3428717