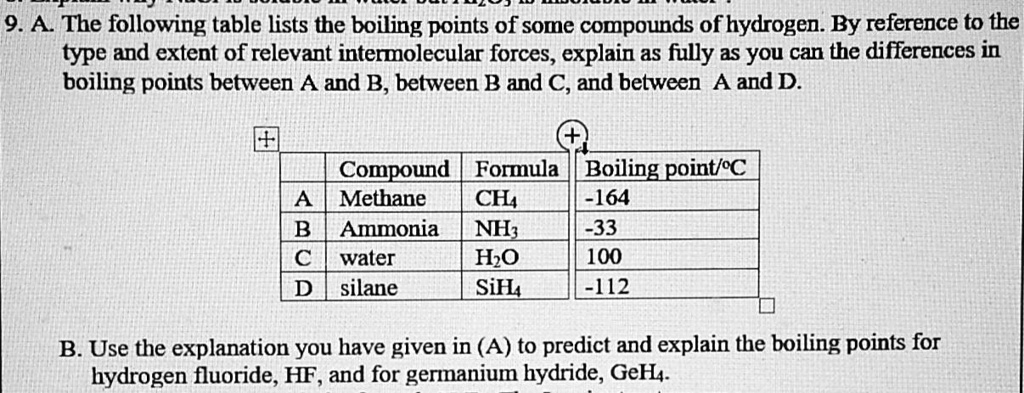
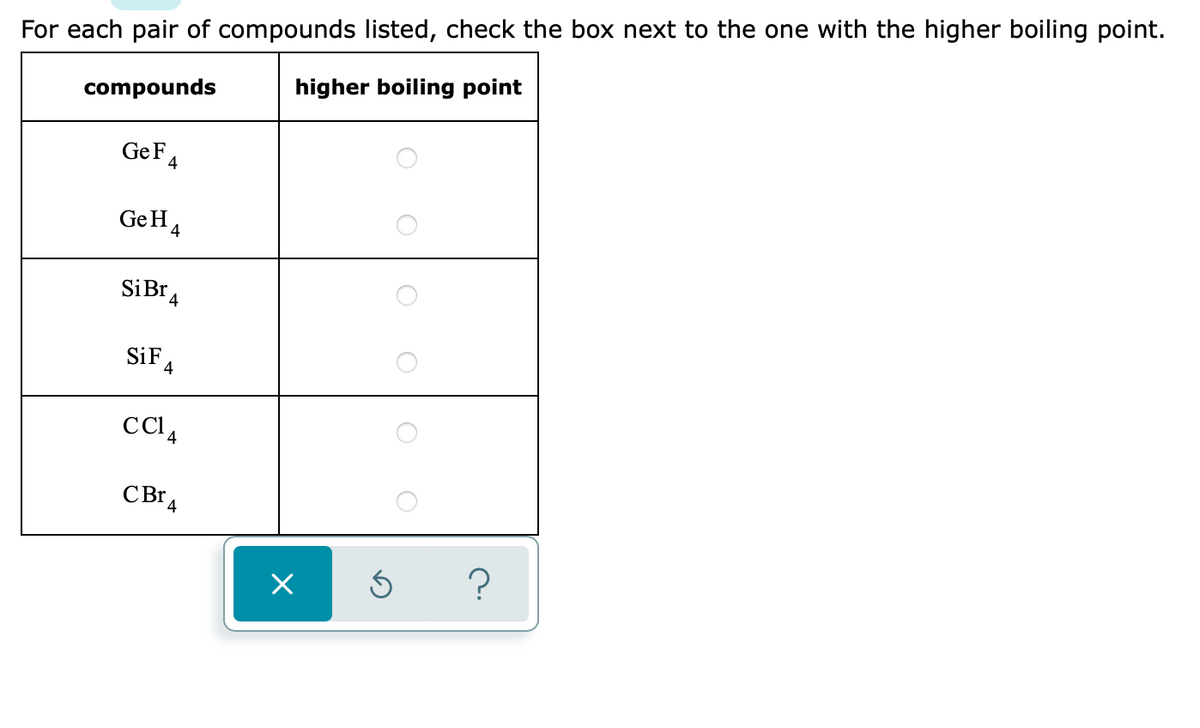
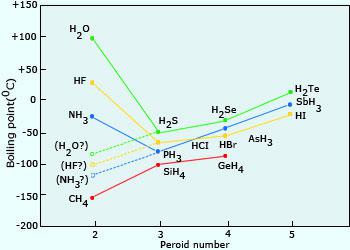
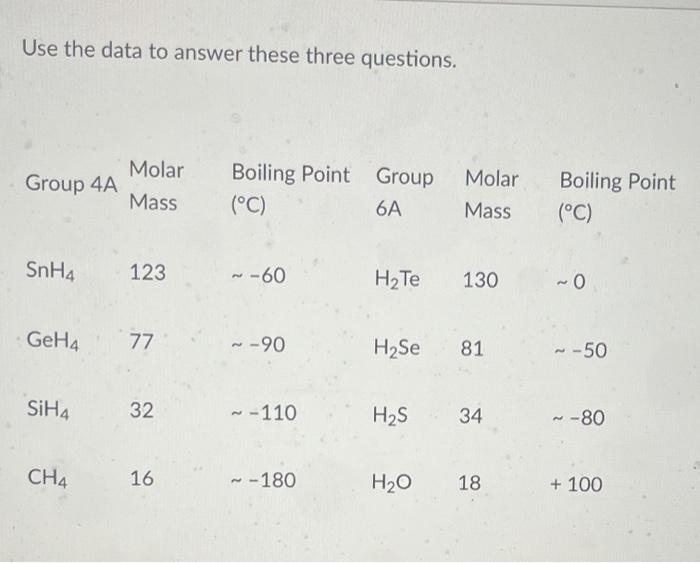
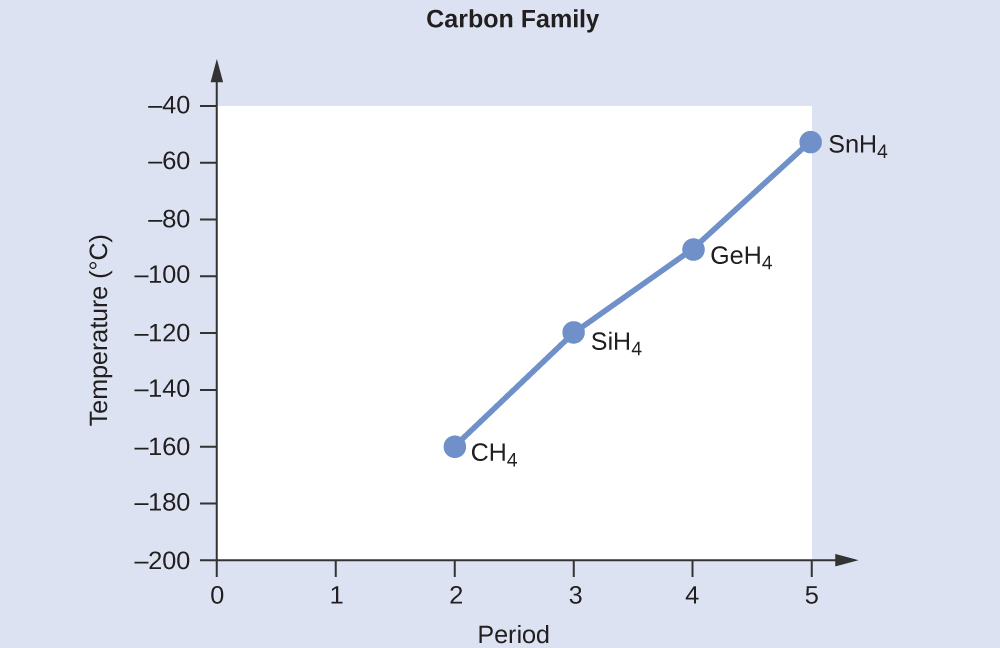
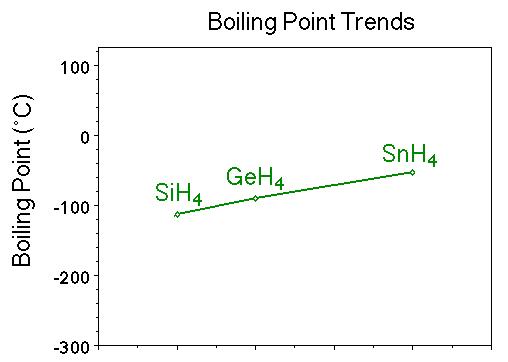
SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘

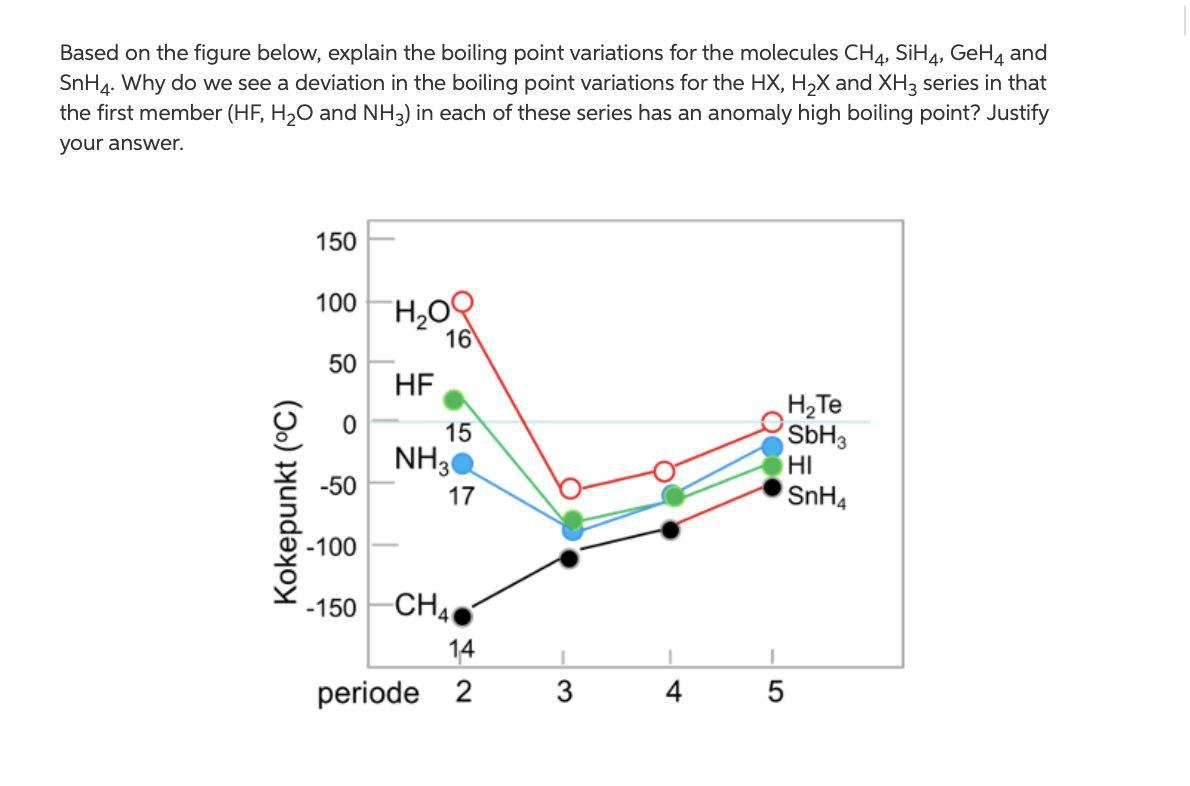
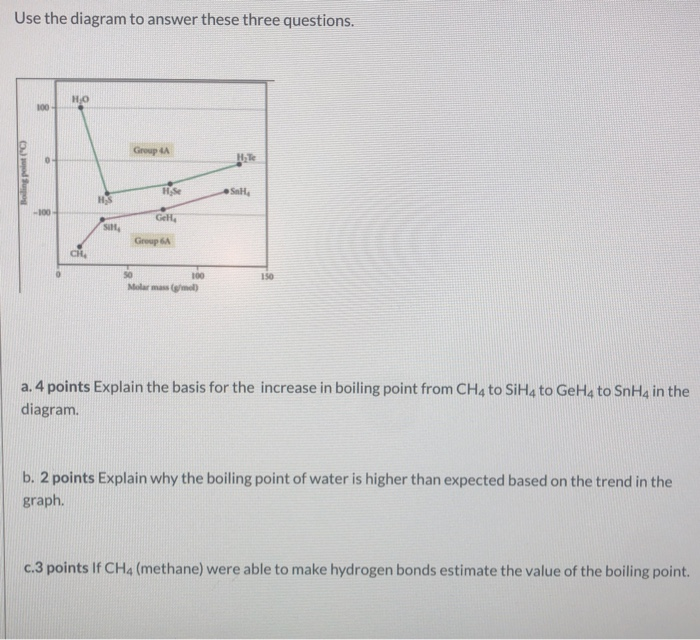
The normal boiling point of water is unusually high, compared to the boiling points of H_2S, H_2Se, and H_2Te. Explain this observation in terms of the hydrogen bonding that exists in water,
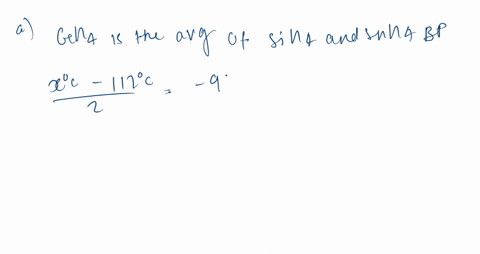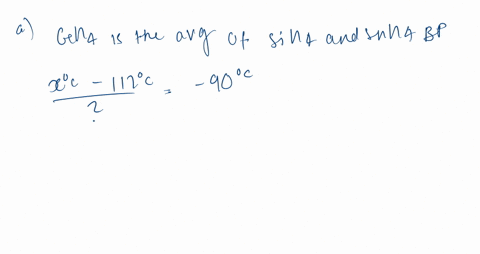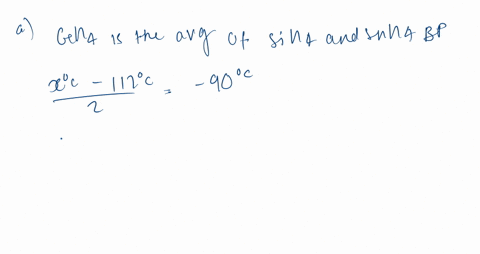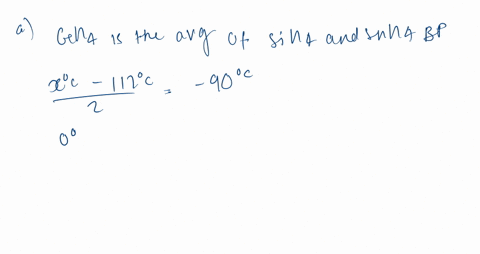
SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘
The correct order for melting point and boiling point of IV group hydrides respectively I) CH(4) lt SiH(4) lt GeH(4) lt SnH(4) II) CH(4) gt SiH(4) lt GeH(4) lt SnH(4) III) SnH(4)