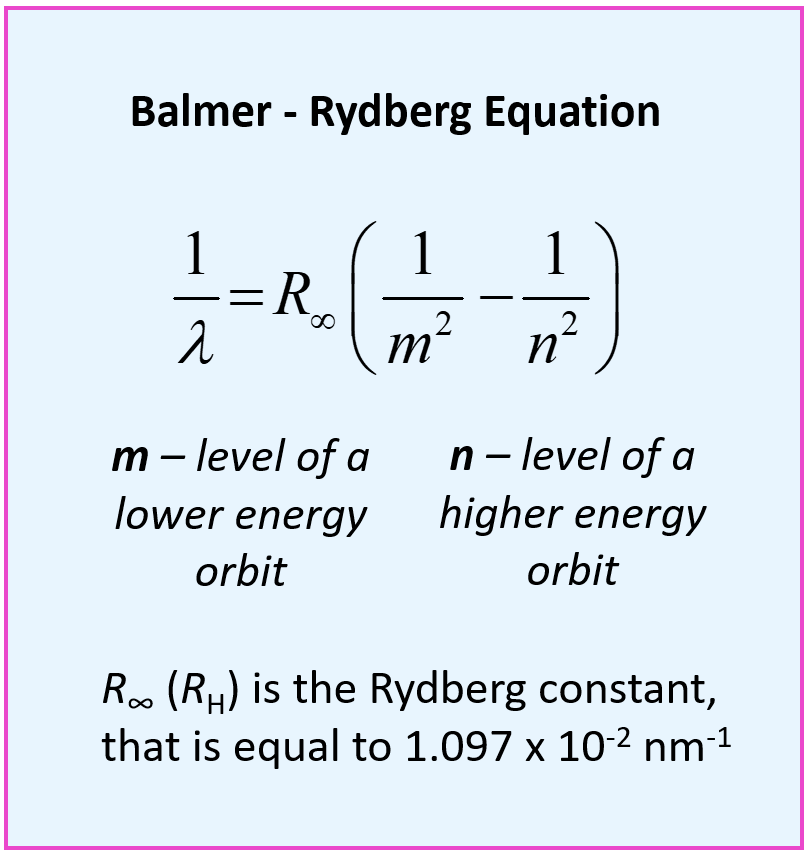
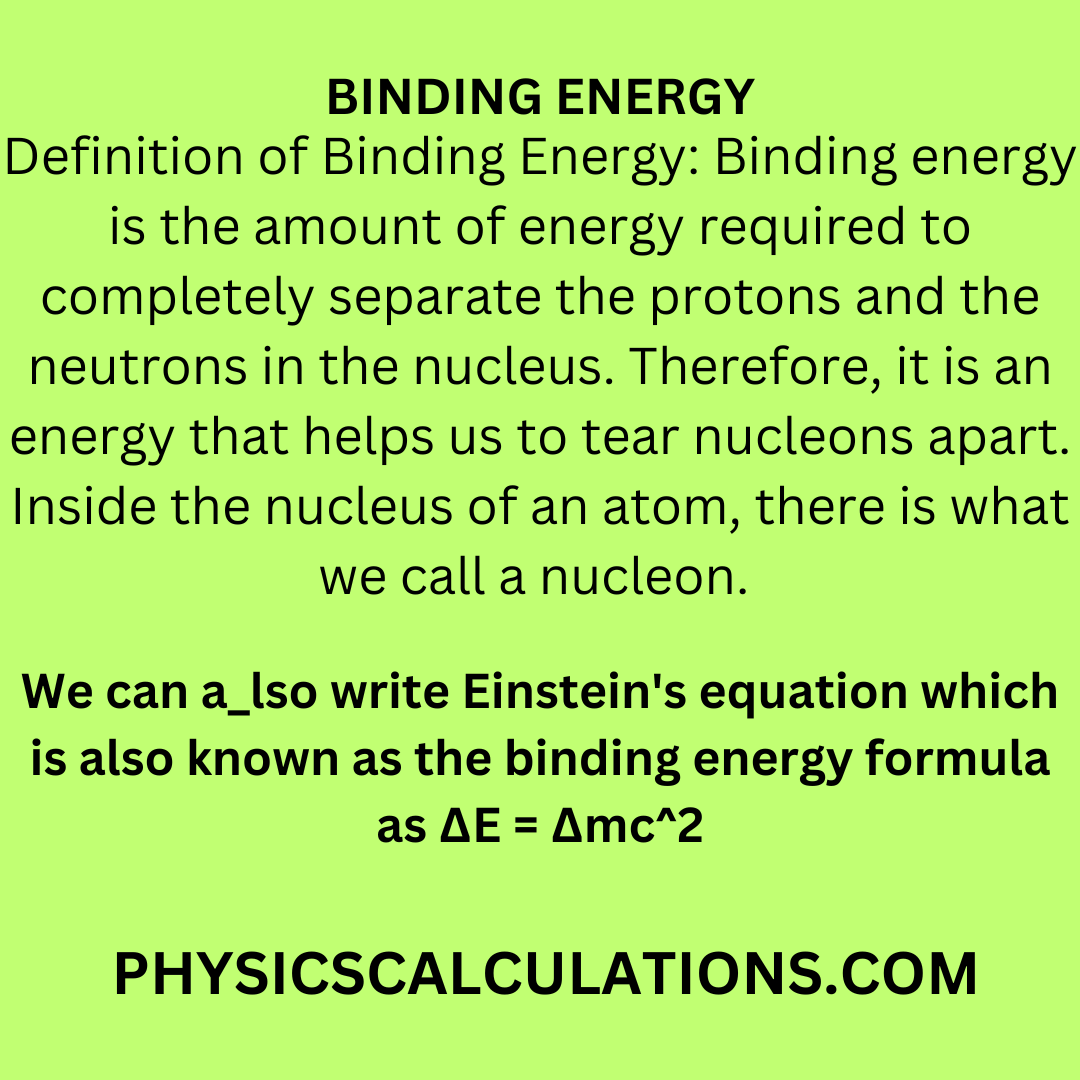
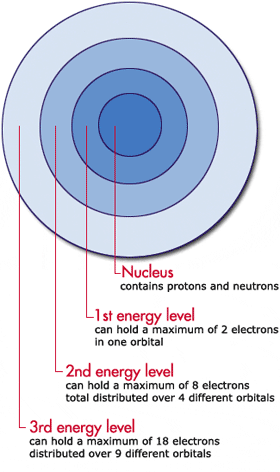
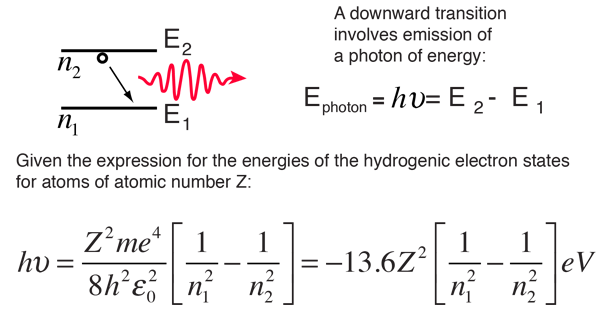
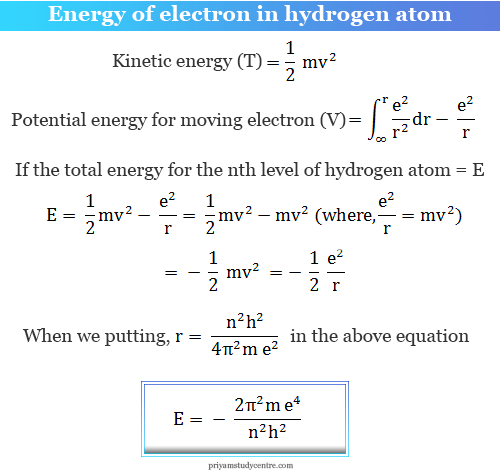
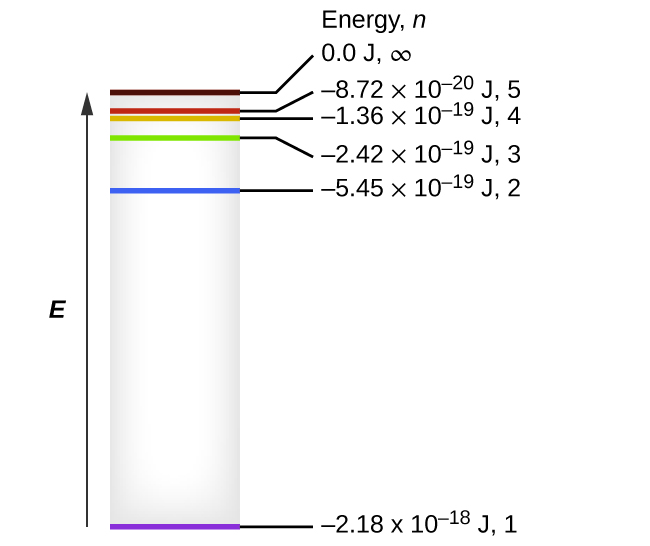
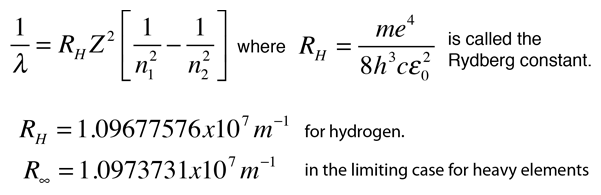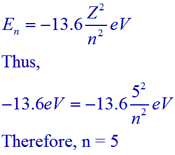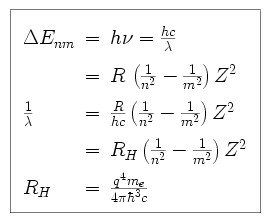What is the formula to calculate the radius of an orbit of the atom and velocity of the specific shell of the atom.

Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Series - YouTube

Electron Energy Level Equations & Examples | What is an Energy Level of an Atom? - Video & Lesson Transcript | Study.com

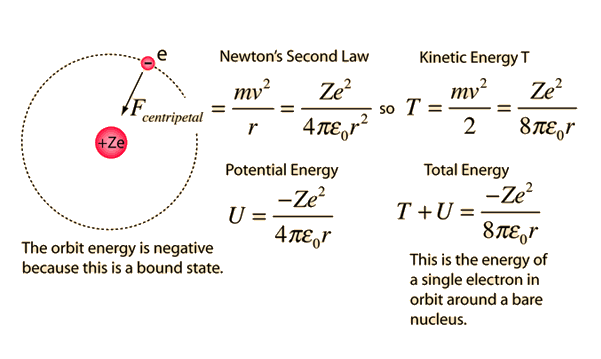
.PNG)