What is the molarity of a final solution after mixing 30 ml of 0.2M HCL and 20 ml of a 0.1M naoh solution? - Quora

Molarity vs. Molality: Examples | How to Calculate Molarity & Molality - Video & Lesson Transcript | Study.com

Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration Problems - YouTube
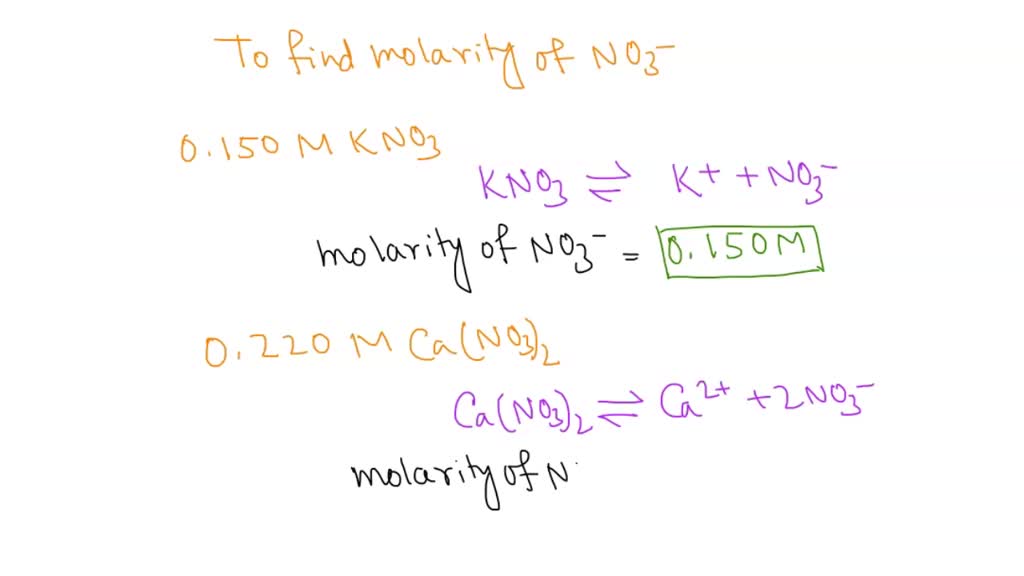
SOLVED: What is the molarity of NO?3 in each solution? 0.150 M KNO3. 0.220 M Ca(NO3)2 0.370 M Al(NO3)3.

Converting molarity to mole fraction, mass percent and molality: A 0.750 M solution of H(2)SO(4) in water has a density of 1.049 g mL^(-1) at 20 ^(@)C. What is the concentration of

Dilution Equation & Examples | How to Calculate Dilution Factors - Video & Lesson Transcript | Study.com

Calculate the molarity of a solution that is 39.77% H2SO4 by mass. The specific gravity of the solution is 1.305. | Homework.Study.com















