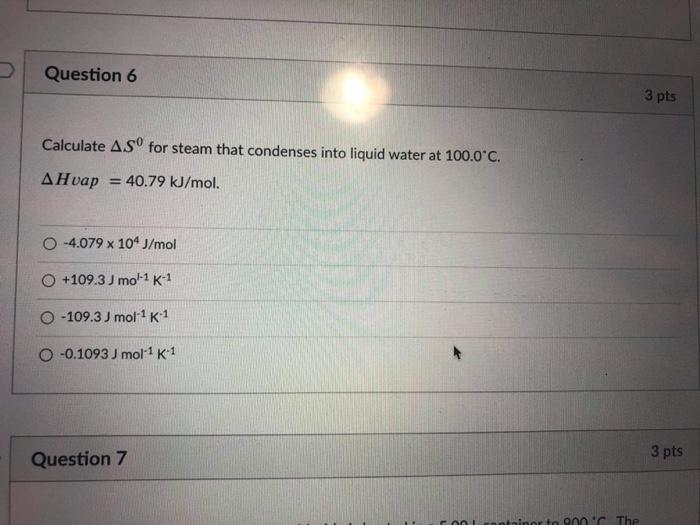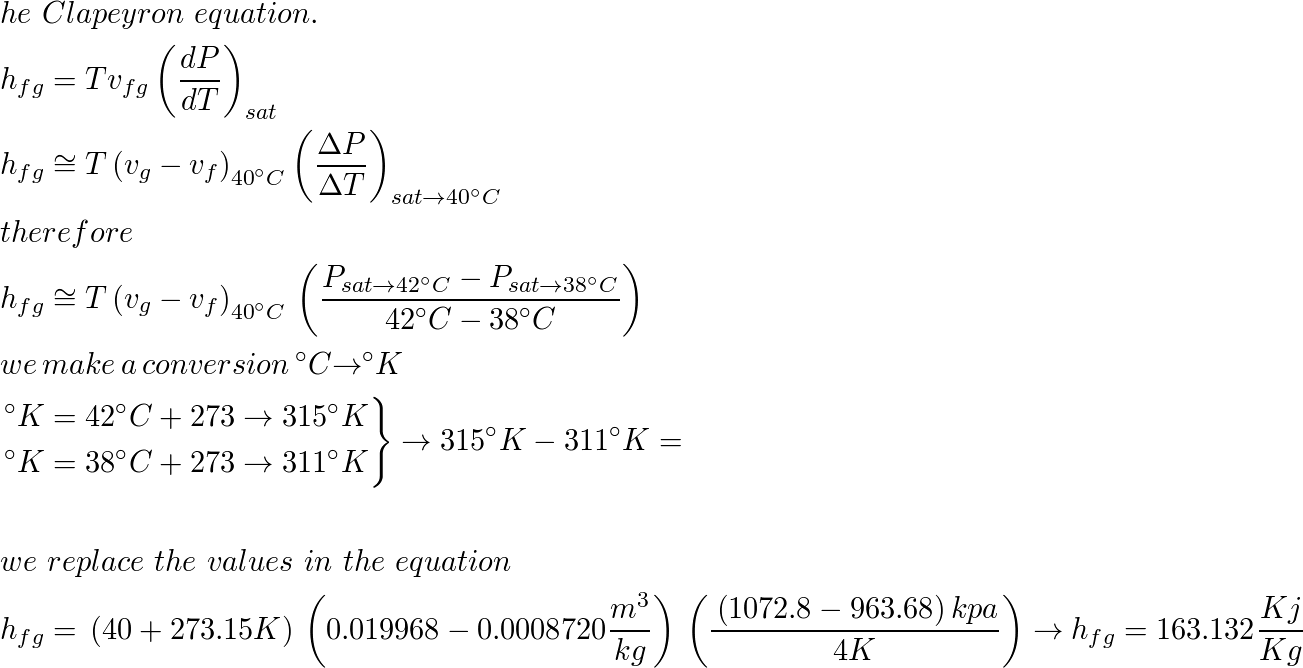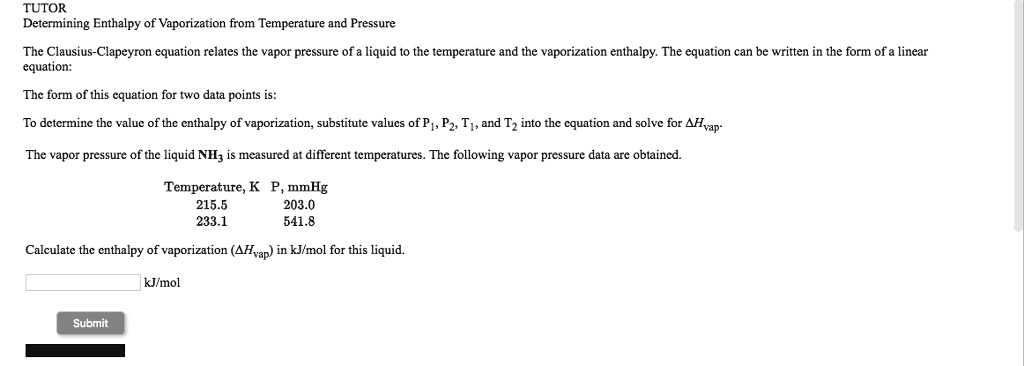
SOLVED: TUTOR Determining Enthalpy of' Vaporization from Terperature and Pressure The Clausius-Clapeyron equation relates the vapor pressure of liquid t0 the ternperalure and the vaporization enthalpy: The equation can be written in

SOLVED: Please use Clausius Clapeyron equation Please do not copy previus answer, i have already solved for a, i need help with b answer should be: a)21,9 kJ/mol and b)92,9 oC 1.
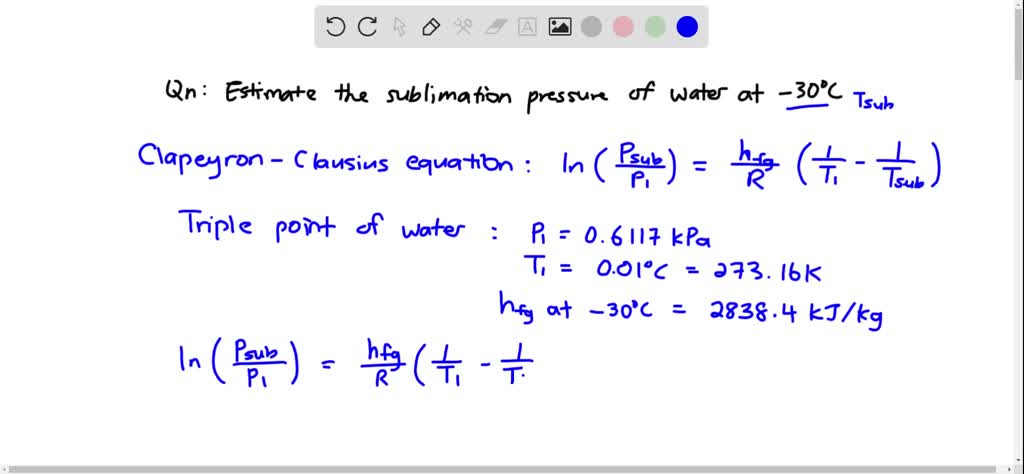
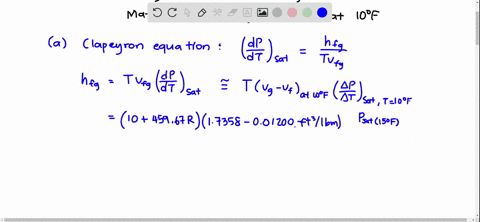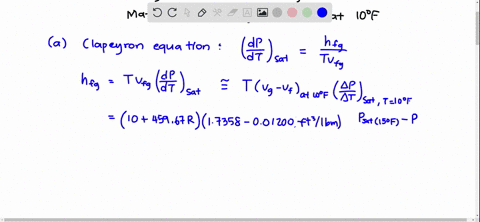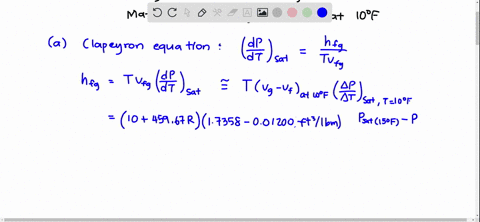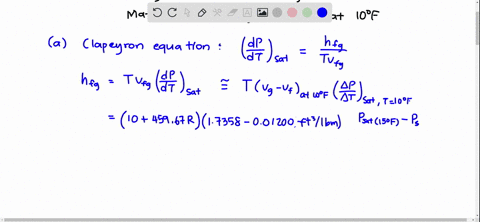
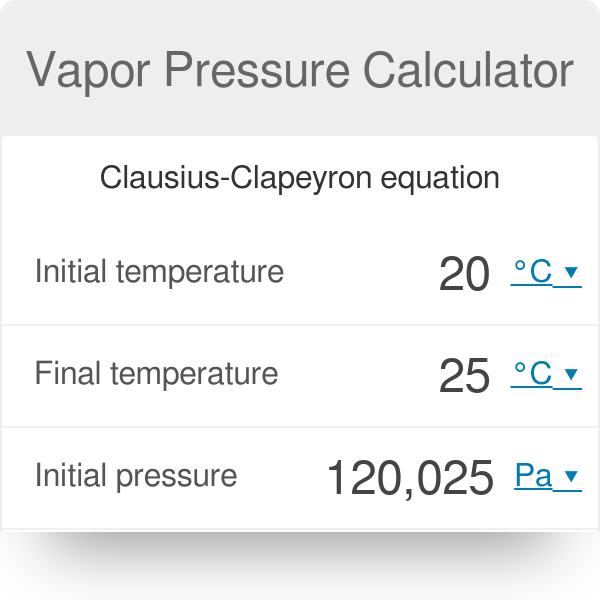
SOLVED:Using the Clapeyron-Clausius equation and the triplepoint data of water, estimate the sublimation pressure of water at -30^∘ C and compare to the yalue in Table A-8.

Vapor Pressure Formula & Example | How to Calculate Vapor Pressure - Video & Lesson Transcript | Study.com

Experimental and calculated by Clausius-Clapeyron equation values of... | Download Scientific Diagram

Characteristic equations for saturated and superheated steam . and included the specific volumes ofsaturated and superheated steam. To determine the validity of Battellis discussion Knob-lauch, Linde, and Klebe-1- conducted a series of

Knowing the vapor pressure of water is 40.7kJ/mol,calculate the vapor pressure of water at 58 degrees Celsius. | Homework.Study.com