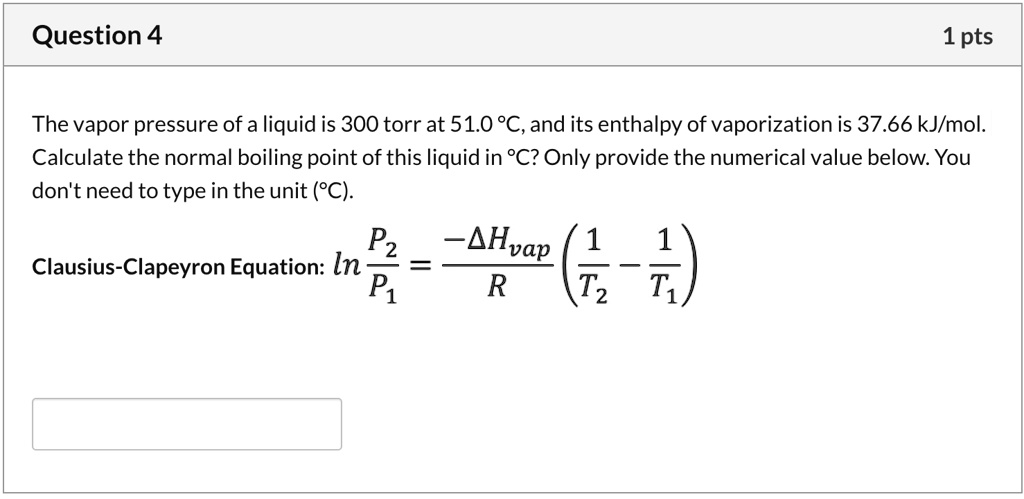
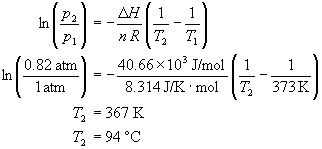Calculate the boiling point of water at 24 torr pressure. The average Hvap. over the temperature range is 10.12 kcal mol^-1 . Will all the water form gaseous state if placed in

Determine the normal boiling point in K of a substance whose vapor pressure is 55.1 mmHg at 23.2^o C and has a ?H_vap of 32.1 kJ/mol. | Homework.Study.com

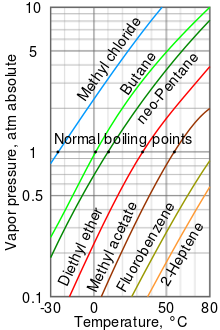
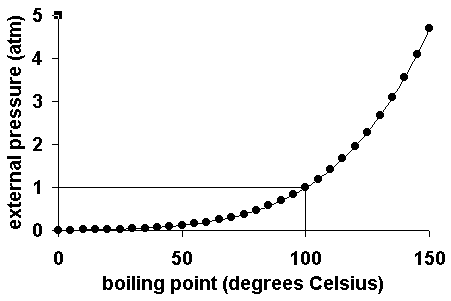
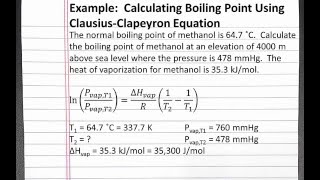
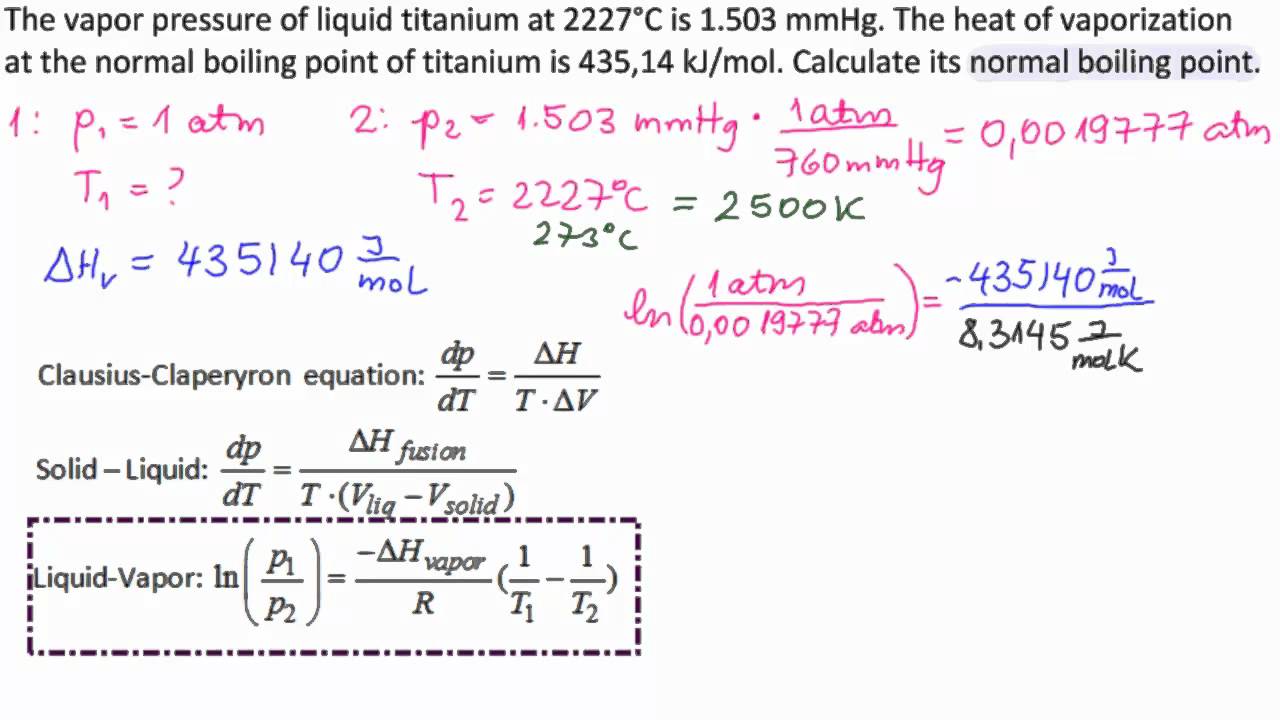
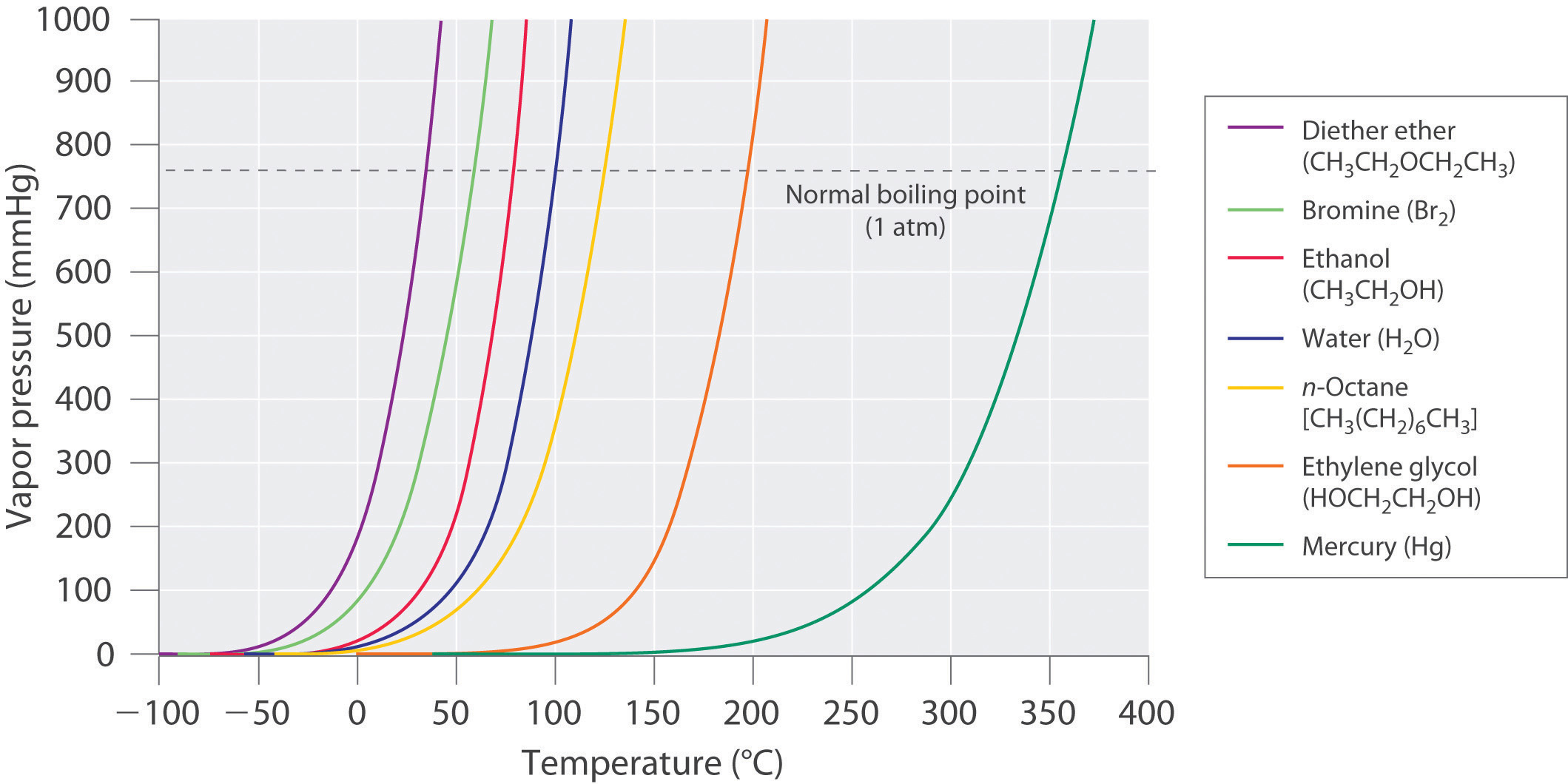
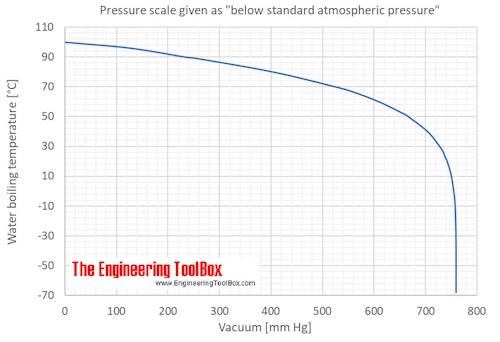
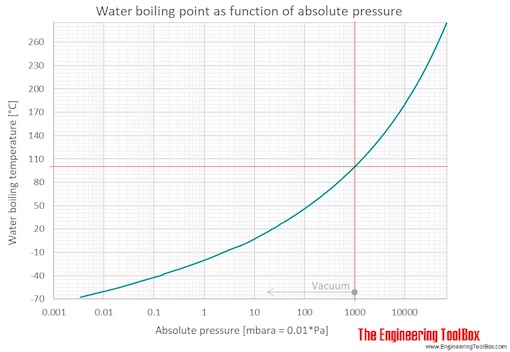
theoretical chemistry - How to calculate melting/boiling points at different pressures? - Chemistry Stack Exchange