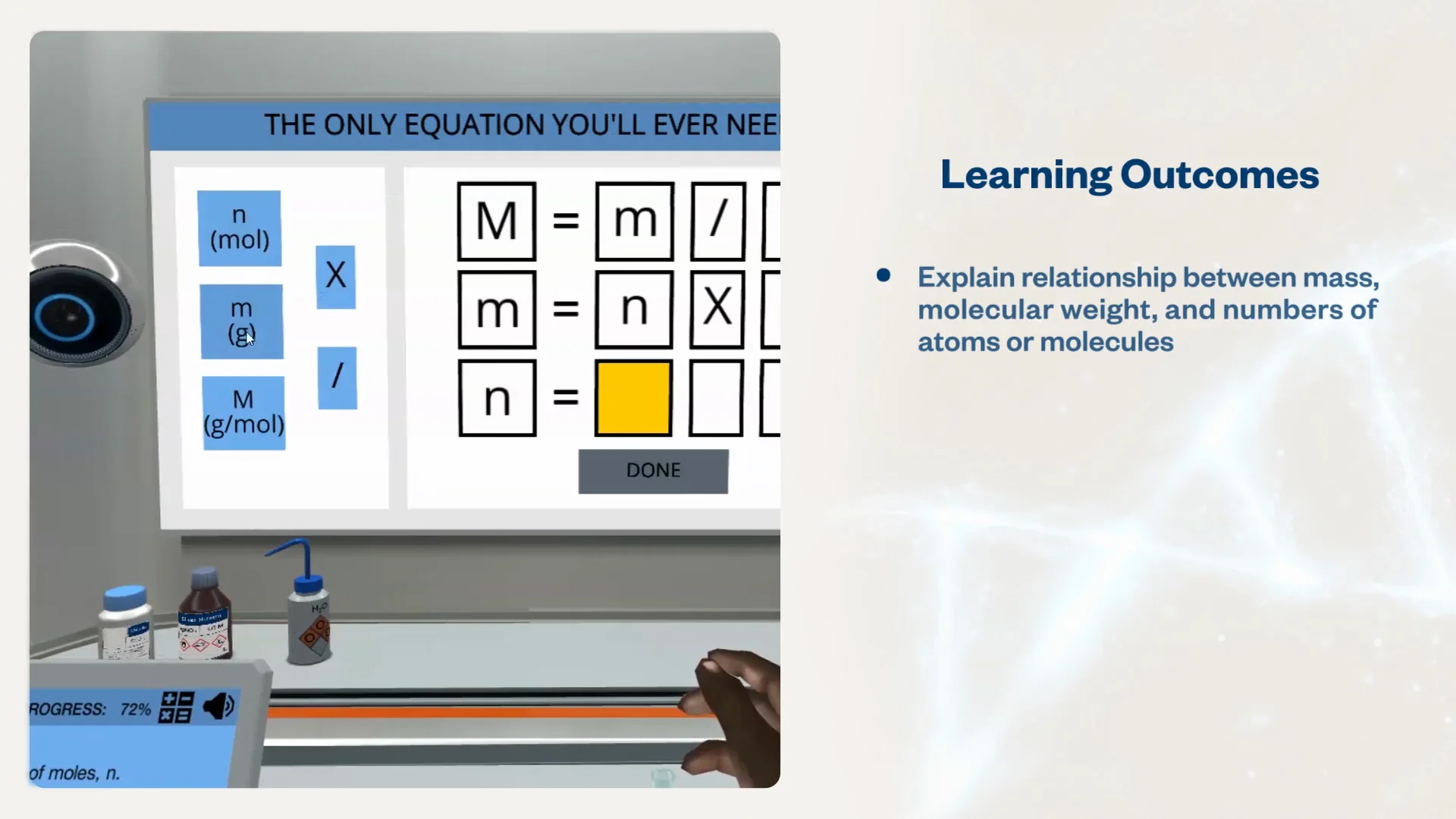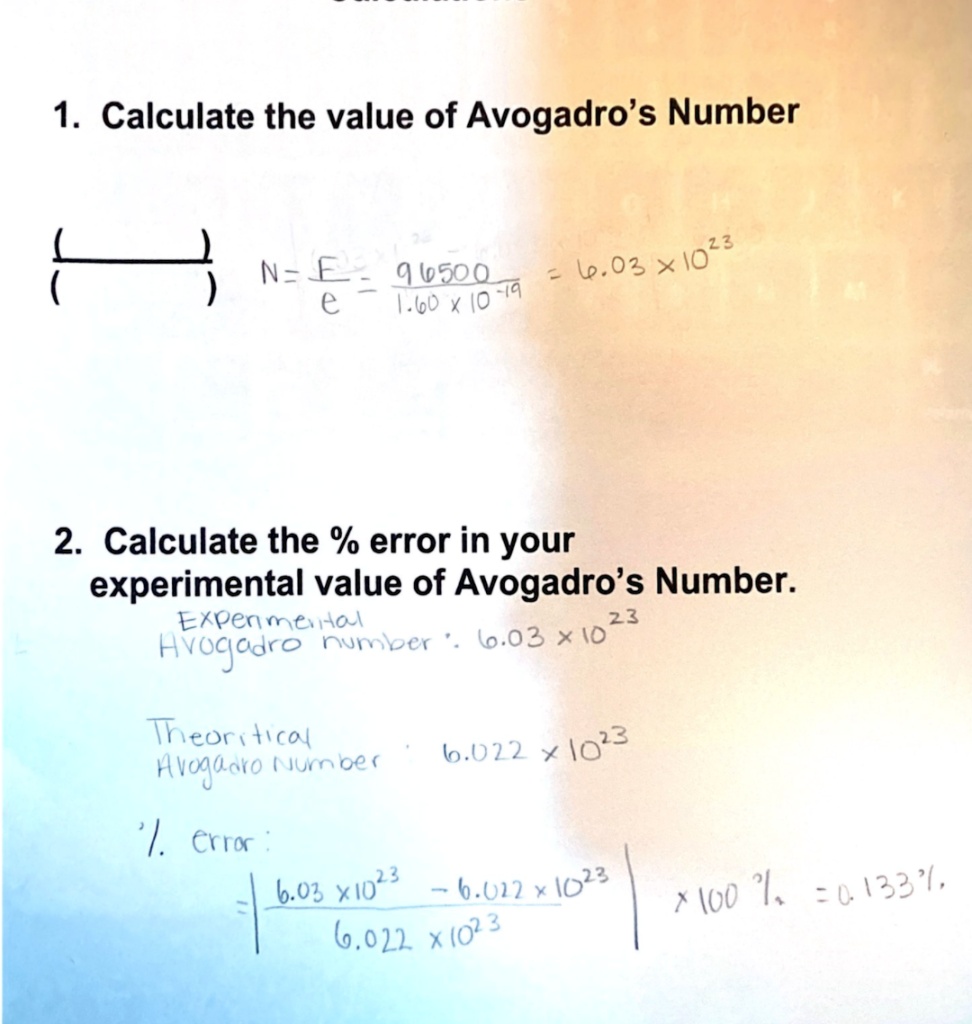
SOLVED: 1 Calculate the value of Avogadro's Number 13 l.03 x10 1 N= € 9.k5oo A.6o / I0 Fiq 2. Calculate the % error in your experimental value of Avogadro's Number: Ekpen

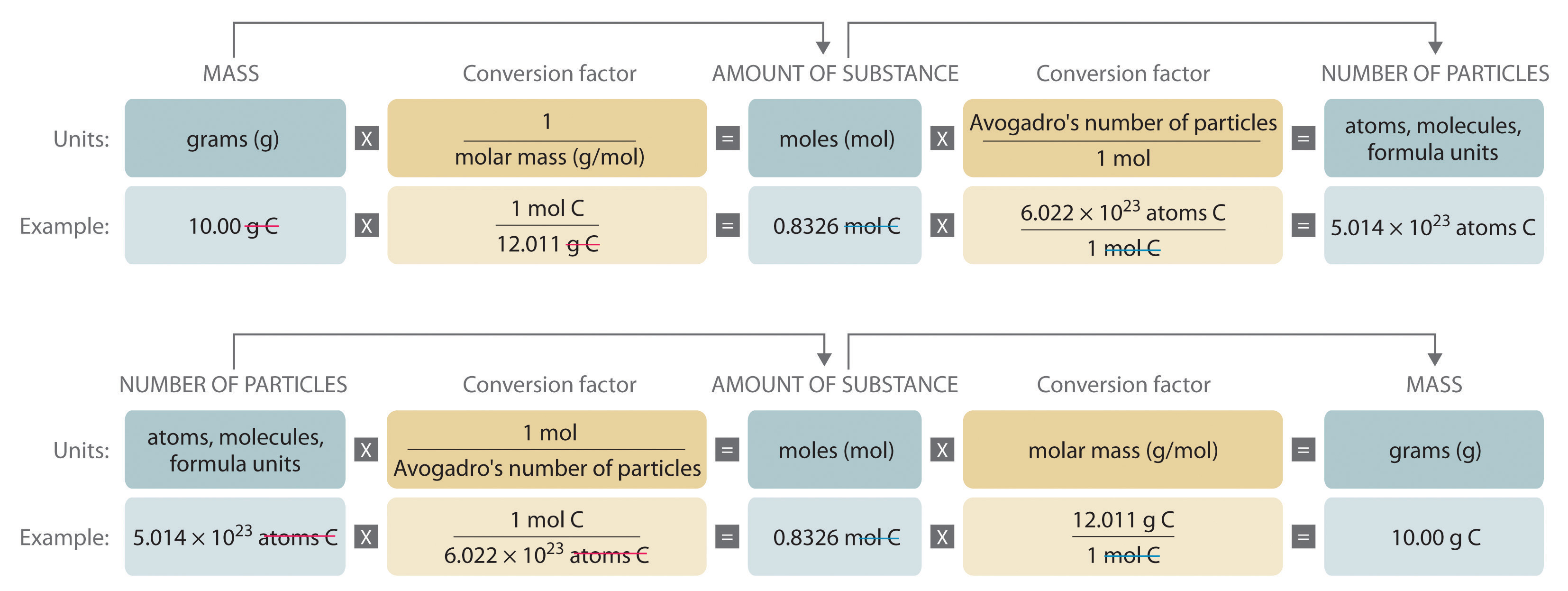
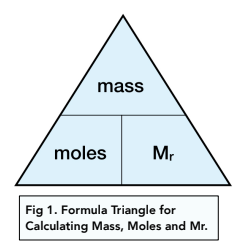
1. Using Avogadro's number, calculate the number of atoms in 0.005 kilograms of carbon. 2. If there are 'x' atoms in 5 grams of carbon, how many atoms are there in 5

Calculate the value of Avogadro number from the internuclear distance of adjacent ions in NaCl , 0.282 nm and the density of solid NaCl is 2.17 × 10^3 kg/m^3 .A unit cell







.PNG)